Au-iClick mirrors the mechanism of copper catalyzed azide–alkyne cycloaddition (CuAAC)†
Abstract
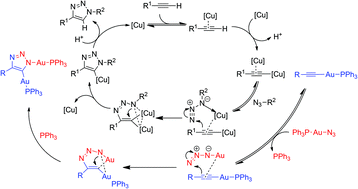
This report outlines the investigation of the iClick mechanism between gold(I)–azides and gold(I)–acetylides to yield digold triazolates. Isolation of digold triazolate complexes offer compelling support for the role of two copper(I) ions in CuAAC. In addition, a kinetic investigation reveals the reaction is first order in both Au(I)–N3 and Au(I)–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–R, thus second order overall. A Hammett plot with a ρ = 1.02(5) signifies electron-withdrawing groups accelerate the cycloaddition by facilitating the coordination of the second gold ion in a π-complex. Rate inhibition by the addition of free triphenylphosphine to the reaction indicates that ligand dissociation is a prerequisite for the reaction. The mechanistic conclusions mirror those proposed for the CuAAC reaction.
C–R, thus second order overall. A Hammett plot with a ρ = 1.02(5) signifies electron-withdrawing groups accelerate the cycloaddition by facilitating the coordination of the second gold ion in a π-complex. Rate inhibition by the addition of free triphenylphosphine to the reaction indicates that ligand dissociation is a prerequisite for the reaction. The mechanistic conclusions mirror those proposed for the CuAAC reaction.

 Please wait while we load your content...
Please wait while we load your content...