Tandem hydrogenation and condensation of fluorinated α,β-unsaturated ketones with primary amines, catalyzed by nickel†
Abstract
A simple homogeneous catalytic system based on nickel phosphine complexes has been developed for the transfer hydrogenation and condensation of α,β-unsaturated ketones to yield saturated ones and saturated imines using primary amines as hydrogen donors. Thus, a wide range of fluorinated 1,5-diaryl-1,4-pentadiene-3-ones were allowed to react with substituted benzylamines in the presence of [(dippe)Ni(μ-H)]2 (dippe = 1,2-bis-(diisopropylphosphino)-ethane) using ethanol as a solvent at 180 °C to give the corresponding saturated carbonyl compounds; here hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond was preferred over the C
C bond was preferred over the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. Under the same reaction conditions but using an excess of benzylamine, a tandem process is then favoured, starting also with the reduction of the C
O bond. Under the same reaction conditions but using an excess of benzylamine, a tandem process is then favoured, starting also with the reduction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond followed by a nucleophilic addition of the primary amine to yield valuable saturated imines with good to excellent yields (62%–91%).
C bond followed by a nucleophilic addition of the primary amine to yield valuable saturated imines with good to excellent yields (62%–91%).
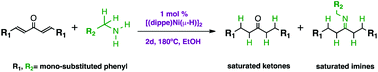

 Please wait while we load your content...
Please wait while we load your content...