Mechanistic understanding of CoO-catalyzed hydrogen desorption from a LiBH4·NH3–3LiH system†
Abstract
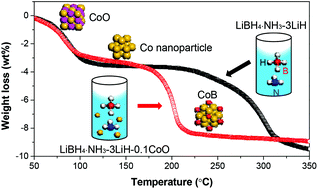
Addition of a minor quantity of CoO significantly reduces the dehydrogenation temperature, accelerates the dehydrogenation rate and increases the hydrogen purity of the LiBH4·NH3–3LiH system. The LiBH4·NH3–3LiH–0.1CoO sample exhibits optimal dehydrogenation properties because it releases 8.5 wt% of hydrogen below 250 °C, which is approximately 90 °C lower than that of the pristine sample. At 200 °C, approximately 8.0 wt% of hydrogen is released from the LiBH4·NH3–3LiH–0.1CoO sample within 100 min, whereas only 4.1 wt% is released from the pristine sample under identical conditions. The EXAFS analyses reveal that upon heating, CoO is first reduced to metallic Co at 130 °C and then partially combines with B to form a Co–B species. The in situ formed Co and Co–B are finely dispersed in the dehydrogenated intermediates, and they play critical roles as active catalysts in favour of breaking the B–H bonds of the Li–B–N–H species. This effectively decreases the thermodynamic and kinetic barriers of the dehydrogenation reaction of the LiBH4·NH3–3LiH system.

 Please wait while we load your content...
Please wait while we load your content...