Formation of ortho-cyano-aminothiophenolate ligands with versatile binding modes via facile carbon–sulfur bond cleavage of 2-aminobenzothiazoles at mercury(ii) centres†
Abstract
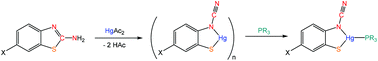
Addition of 2-aminobenzothiazole and substituted derivatives to mercuric acetate in warm ethanol leads to the high yield formation of [Hg{SC6H3XN(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)}]n resulting from loss of hydrogen and sulfur–carbon bond cleavage. Addition of phosphines affords a series of complexes in which the new ortho-cyano-aminothiophenolate ligands adopt three different binding modes.
N)}]n resulting from loss of hydrogen and sulfur–carbon bond cleavage. Addition of phosphines affords a series of complexes in which the new ortho-cyano-aminothiophenolate ligands adopt three different binding modes.

 Please wait while we load your content...
Please wait while we load your content...