DFT analysis into the intermediates of nickel pyridinethiolate catalysed proton reduction†
Abstract
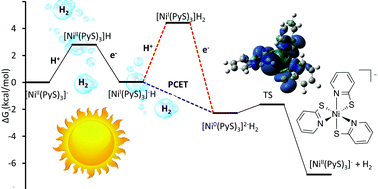
Nickel pyridine 2-thiolate (Ni(PyS)3−) has shown good stability and activity as a H2 generation catalyst for use in solar energy storage. The experimentally proposed catalytic pathway is explored using DFT calculations. Free energy changes along the reaction coordinate, spin states, localization of charge and geometry of the intermediates were explored. Calculations were performed using Gaussian 09 with a B3P86/6-31+G(d) basis set and a CPCM water solvation model. Of particular interest were our findings that the first reduction occurs at the nickel rather than through non-innocent ligands and that water coordination is not favourable although protonation of the pyridyl nitrogen causes dechelation. Sequential and concerted proton coupled electron transfer were considered in the formation of the hydride.


 Please wait while we load your content...
Please wait while we load your content...