Alkoxy substituted halogenated closo-dodecaborates as anions for ionic liquids†
Abstract
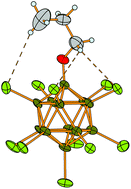
Halogenated and alkoxylated closo-dodecaborates [B12X11OR]2− (X = Cl, Br; R = propyl, octyl, dodecyl) have been synthesized by halogenation of the known [B12H11OH]2− anion followed by alkylation in the superbasic medium DMSO/KOH. The obtained sodium salts were transformed by simple metathesis reactions in aqueous solution to the tetrabutylammonium ([NBu4]+) and 1-hexyl-3-methylimidazolium ([C6mim]+) salts. All compounds were fully characterized by heteronuclear NMR, IR and Raman spectroscopy, ESI mass spectrometry, and thermal analytical measurements. Selected anions were also structurally characterized as their [Ph4P]+ salts by single crystal X-ray diffraction. The [C6mim]+ salts are thermally stable up to more than 300 °C and show clear melting points. Surprisingly, the compound [C6mim]2[B12Cl11O-propyl] having the short propyl group bound to the boron cluster shows the lowest melting point (96 °C) of all the investigated compounds. Thus this compound is a rare member of the class of ionic liquids consisting of dianions.

 Please wait while we load your content...
Please wait while we load your content...