Non-nitric oxide based metallovasodilators: synthesis, reactivity and biological studies†
Abstract
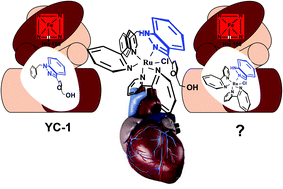
There is an increasing number of compounds developed to target one or more pathways involved in vasodilation. Some studies conducted with azaindole and indazole derivatives showed cardiovascular activity associated with these compounds. Fast and easy structural modification of these organic molecules can be achieved using metal complexes promoting a much larger spatial change than organic strategies, potentially leading to novel drugs. Here, we have prepared a series of complexes with a formula cis-[RuCl(L)(bpy)2]PF6, where L = 7-azaindole (ain), 5-azaindole (5-ain), 4-azaindole (4-ain), indazole (indz), benzimidazole (bzim) or quinoline (qui), which were characterized by spectroscopic and electrochemical techniques (CV, DPV). These compounds showed reasonable stability exhibiting photoreactivity only at low wavelength along with superoxide scavenger activity. Cytotoxicity assays indicated their low activity preliminarily supporting in vivo application. Interestingly, vasodilation assays conducted in rat aorta exhibited great activity that largely improved compared to free ligands and even better than the well-studied organic compound (BAY 41-42272), with IC50 reaching 55 nM. These results have validated this strategy opening new opportunities to further develop cardiovascular agents based on metallo-bicyclic rings.

 Please wait while we load your content...
Please wait while we load your content...