Impact of SDS surfactant on the interactions of Cu2+ ions with the amyloidogenic region of human prion protein†
Abstract
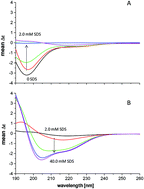
Prion diseases, known as Transmissible Spongiform Encephalopathies (TSEs), are a group of fatal neuronal, and to some extent infectious disorders, associated with a pathogenic protein agent called prion protein (PrP). The human prion protein (hPrP) fragment encompassing the 91–127 region, also known as the amyloidogenic domain, comprises two copper-binding sites corresponding to His-96 and His-111 residues that act as anchors for Cu2+ binding. In this work, we investigated Cu2+ interaction with hPrP91–127 in the presence of the anionic surfactant sodium dodecyl sulfate (SDS), which induces a partial α-helix folding of the peptide. Our data indicate that the Cu2+ coordination ability of the amyloidogenic fragment in the presence of SDS micelles is significantly different to that observed in aqueous solution. This is mainly due to the fact that SDS micelles strongly stabilize the formation of the α-helical structure of the peptide backbone, which is well conserved also upon Cu2+ binding, contrary to the random coil conformation mainly assumed by hPrP91–127 in aqueous solutions. Potentiometric and spectroscopic studies clearly indicate that in the case of SDS containing solutions, Cu2+ ions coordinate simultaneously to both imidazoles, while in the case of water solutions, metal ion coordination involves only a single His side chain, which individually acts as an independent Cu2+ anchoring site.

 Please wait while we load your content...
Please wait while we load your content...