The crystal structure and magnetic properties of 3-pyridinecarboxylate-bridged Re(ii)M(ii) complexes (M = Cu, Ni, Co and Mn)†
Abstract
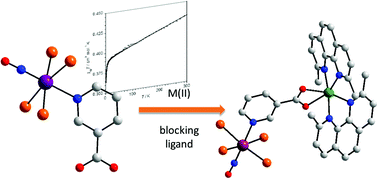
The novel Re(II) complex NBu4[Re(NO)Br4(Hnic)] (1) and the heterodinuclear compounds [Re(NO)Br4(μ-nic)Ni(dmphen)2]·½CH3CN (2), [Re(NO)Br4(μ-nic)Co(dmphen)2]·½MeOH (3), [Re(NO)Br4(μ-nic)Mn(dmphen)(H2O)2]·dmphen (4), [Re(NO)Br4(μ-nic)Cu(bipy)2] (5) [Re(NO)Br4(μ-nic)Cu(dmphen)2] (5′) (NBu4+ = tetra-n-butylammonium cation, Hnic = 3-pyridinecarboxylic acid, dmphen = 2,9-dimethyl-1,10-phenanthroline, bipy = 2,2′-bipyridine) have been prepared and the structures of 1–5 determined using single crystal X-ray diffraction. The structure of 1 consists of [Re(NO)Br4(Hnic)]− anions and NBu4+ cations. Each Re(II) is six-coordinate with four bromide ligands, a linear nitrosyl group and a nitrogen atom from the Hnic molecule, in a distorted octahedral surrounding. The structures of 2–5 are made up of discrete heterodinuclear Re(II)M(II) units where the fully deprotonated [Re(NO)Br4(nic)]2− entity acts as a didentate ligand through the carboxylate group towards the [Ni(dmphen)2]2+ (2), [Co(dmphen)2]2+ (3), [Mn(dmphen)(H2O)2]2+ (4) and [Cu(bipy)2]2+ (5) fragments, the Re–M separation across the nic bridge being 7.8736(8) (2), 7.9632(10) (3), 7.7600(6) (4) and 8.2148(7) Å (5). The environment of the Re(II) ion in 2–5 is the same as that in 1 and all M(II) are six-coordinate in highly distorted octahedral surroundings, the main source of the distortion being due to the reduced bite of the chelating carboxylate. The magnetic properties of 1–5′ were investigated in the temperature range 1.9–300 K. 1 behaves as a quasi-magnetically isolated spin doublet with very weak antiferromagnetic interactions through space Br⋯Br contacts. Its magnetic susceptibility data were successfully modeled through a deep analysis of the influence of the ligand field, spin–orbit coupling, tetragonal distortion and covalence effects as variable parameters. Compounds 2–5′ exhibit weak antiferromagnetic interactions. The intramolecular exchange pathway in this family being discarded because of the symmetry of magnetic orbitals of the Re(II) ion (dxy) precludes any spin delocalization on the bridging nic orbitals, the observed magnetic interactions are most likely mediated by π–π type interactions between the peripheral ligands which occur in them. Only in the case of 4, short through space Br⋯Br contacts of ca. 4.03 Å (values larger than 5.5 Å in 2, 3 and 5) could be involved in the exchange coupling.

 Please wait while we load your content...
Please wait while we load your content...