Imido-pyridine Ti(iv) compounds: synthesis of unusual imido–amido heterobimetallic derivatives†
Abstract
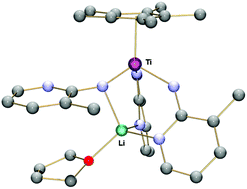
The reaction of lithiated picolines and [TiCl3(η5-C5Me5)] leads to several bridging or terminal imido compounds, each of which can be selectively formed by controlling the stoichiometry and temperature. Specifically, the dinuclear imido-bridged [TiCl(η5-C5Me5)(μ-NR)]2 (1a, NR = 2-imido-3-picoline; 1b, NR = 2-imido-5-picoline) species and the unusual Ti–Li imido–amido heterobimetallic complex [{Li(THF)}{Ti(η5-C5Me5)(NR)(NHR)2}] (2a, NR = 2-imido-3-picoline; 2b, NR = 2-imido-5-picoline) were isolated. Compounds 2 are in effect the first structurally characterized examples of titanium(IV) coordinated to terminal imido-pyridines. DFT-D calculations for 2a denote a multiple bond character between titanium and the imido ligand and a strong polarization of the electron density by the alkali cation in spite of the lack of intermetallic bonding.

 Please wait while we load your content...
Please wait while we load your content...