Oxaliplatin vs. cisplatin: competition experiments on their binding to lysozyme†
Abstract
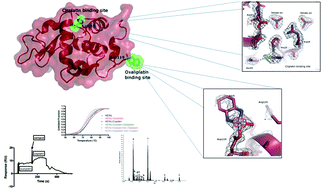
The model protein hen egg white lysozyme was challenged with oxaliplatin and cisplatin. ESI mass spectrometry, surface plasmon resonance and thermal shift analyses demonstrate the formation of a bis-platinum adduct, though in very small amounts. Crystals of the bis-platinum adduct were obtained using two different preparations and the X-ray structures were solved at 1.85 Å and 1.95 Å resolution. Overall, the obtained data point out that, under the analyzed conditions, the two Pt drugs have similar affinities for the protein, but bind on its surface at two non-overlapping sites. In other words, these two drugs manifest a significantly different reactivity with this model protein and do not compete for the same protein binding sites.

 Please wait while we load your content...
Please wait while we load your content...