Catalytic catechol oxidation by copper complexes: development of a structure–activity relationship†
Abstract
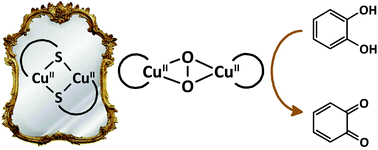
A large library of CuII complexes with mononucleating and dinucleating ligands was synthesized to investigate their potential as catalysts for the catalytic oxidation of 3,5-di-tert-butylcatechol (3,5-DTBC). X-ray structure determination for a number of these complexes revealed relatively large Cu⋯Cu distances and the formation of polymeric species. Comparison of the 3,5-DTBC oxidation rates showed that ligands that stabilize the biomimetic dinuclear CuII μ-thiolate complex also result in copper compounds that are much more active in the oxidation of 3,5-DTBC. This oxidation activity is however inhibited by the presence of chloride ions. The highest kcat that was observed was 6900 h−1, which is one of the highest turnover frequencies reported so far for catechol oxidation in CH3CN.
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...