Alluaudite Na2Co2Fe(PO4)3 as an electroactive material for sodium ion batteries†
Abstract
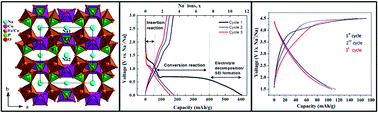
The electroactive orthophosphate Na2Co2Fe(PO4)3 was synthesized using a solid state reaction. Its crystal structure was solved using the combination of powder X-ray- and neutron-diffraction data. This material crystallizes according to the alluaudite structure (S.G. C2/c). The structure consists of edge sharing [MO6] octahedra (M = Fe, Co) resulting in chains parallel to [−101]. These chains are linked together via the [PO4] tetrahedra to form two distinct tunnels in which sodium cations are located. The electrochemical properties of Na2Co2Fe(PO4)3 were evaluated by galvanostatic charge–discharge cycling. During the first discharge to 0.03 V, Na2Co2Fe(PO4)3 delivers a specific capacity of 604 mA h g−1. This capacity is equivalent to the reaction of more than seven sodium ions per formula unit. Hence, this is a strong indication of a conversion-type reaction with the formation of metallic Fe and Co. The subsequent charge and discharge involved the reaction of fewer Na ions as expected for a conversion reaction. When discharged to 0.9 V, the material intercalated only one Na+-ion leading to the formation of a new phase Na3Co2Fe(PO4)3. This phase could then be cycled reversibly with an average voltage of 3.6 V vs. Na+/Na and a capacity of 110 mA h g−1. This result is in good agreement with the theoretical capacity expected from the extraction/insertion of two sodium atoms in Na3Co2Fe(PO4)3.


 Please wait while we load your content...
Please wait while we load your content...