Ruthenium indenylidene complexes bearing N-alkyl/N-mesityl-substituted N-heterocyclic carbene ligands†
Abstract
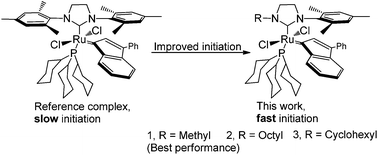
We report on the synthesis and characterization of second generation ruthenium indenylidene catalysts bearing unsymmetrical N-heterocyclic carbene (NHC) ligands denoted as RuCl2(3-phenyl-1-indenylidene)(1-mesityl-3-R-4,5-dihydroimidazol-2-ylidene)(PCy3), in which R is methyl 8a, octyl 8b or cyclohexyl 8c. The characterization of 8a–c was performed by NMR spectroscopy, elemental analysis, IR, HRMS and single-crystal X-ray diffraction analysis. In addition, the catalytic activity of the obtained initiators was evaluated in various representative metathesis reactions. The results reveal that the complexes 8a–c, bearing an N-alkyl side on the NHC, show a faster catalytic initiation than the reference complex 2. Complex 8a, which performs the best among the investigated indenylidene complexes, exhibits slower initiation but better overall efficiency than its benzylidene analogue 1c, especially in a low catalyst loading.

 Please wait while we load your content...
Please wait while we load your content...