Fluorescent mixed ligand copper(ii) complexes of anthracene-appended Schiff bases: studies on DNA binding, nuclease activity and cytotoxicity†
Abstract
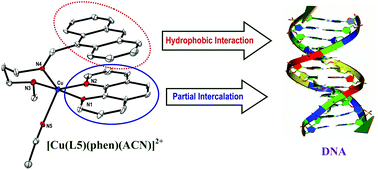
A series of mixed ligand copper(II) complexes of the type [Cu(L)(phen)(ACN)](ClO4)21–5, where L is a bidentate Schiff base ligand (N1-(anthracen-10-ylmethylene)-N2-methylethane-1,2-diamine (L1), N1-(anthracen-10-ylmethylene)-N2,N2-dimethylethane-1,2-diamine (L2), N1-(anthracen-10-yl-methylene)-N2-ethylethane-1,2-diamine (L3), N1-(anthracen-10-ylmethylene)-N2,N2-diethylethane-1,2-diamine (L4) and N1-(anthracen-10-ylmethylene)-N3-methylpropane-1,3-diamine (L5)) and phen is 1,10-phenanthroline, have been synthesized and characterized by spectral and analytical methods. The X-ray crystal structure of 5 reveals that the coordination geometry around Cu(II) is square pyramidal distorted trigonal bipyramidal (τ, 0.76). The corners of the trigonal plane of the geometry are occupied by the N2 nitrogen atom of phen, the N4 nitrogen atom of L5 and the N5 nitrogen of acetonitrile while the N1 nitrogen of phen and the N3 nitrogen of L5 occupy the axial positions with an N1–Cu1–N3 bond angle of 176.0(3)°. All the complexes display a ligand field band (600–705 nm) and three less intense anthracene-based bands (345–395 nm) in solution. The Kb values calculated from absorption spectral titration of the complexes (π → π*, 250–265 nm) with Calf Thymus (CT) DNA vary in the order 5 > 4 > 3 > 2 > 1. The fluorescence intensity of the complexes (520–525 nm) decreases upon incremental addition of CT DNA, which reveals the involvement of phen rather than the appended anthracene ring in partial DNA intercalation with the DNA base stack. The extent of quenching is in agreement with the DNA binding affinities and the relative increase in the viscosity of DNA upon binding to the complexes as well. Thus 5 interacts with DNA more strongly than 4 on account of the stronger involvement in hydrophobic DNA interaction of the anthracenyl moiety, which is facilitated by the propylene ligand backbone with chair conformation. The ability of complexes (100 μM) to cleave DNA (pUC19 DNA) in 5 mM Tris-HCl/50 mM NaCl buffer at pH 7.1 in the absence of a reducing agent or light varies in the order 5 > 4 > 3 > 2 > 1, which is in conformity with their DNA binding affinities. Interestingly, cytotoxicity studies on the MCF-7 human breast cancer cell line show that the IC50 value of 5 is less than that of cisplatin for the same cell line, revealing that it can act as an effective cytotoxic drug in a time-dependent manner.

 Please wait while we load your content...
Please wait while we load your content...