Water-assisted proton delivery and removal in bio-inspired hydrogen production catalysts†
Abstract
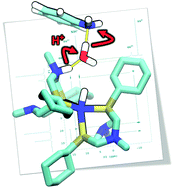
Electrocatalysts for H2 production are envisioned to play an important role in renewable energy utilization systems. Nickel-based catalysts featuring pendant amines functioning as proton relays in the second coordination sphere of the metal center have led to catalysts achieving turnover frequencies as high as 107 s−1 for H2 production. The fastest rates are observed when water is present in solution, with rates up to 103 times faster than those found in dry solvent. The focus of this paper is to provide mechanistic insight into the unexpected enhancement due to water. Addition of H2 to [Ni(PCy2NR′2)2]2+ was previously shown to give three isomers of a Ni(0) product with two protonated amines, where the N–H can be endo or exo to the Ni. By investigating the deprotonation of these two N-protonated Ni(0) intermediates resulting from the addition of H2 to [Ni(PCy2NR′2)2]2+, we observe by NMR spectroscopy studies an enhancement in the rate of deprotonation for protons positioned on the pendant amine next to the metal (endo) vs. protons that are positioned away from the metal (exo). Computational studies suggest that for smaller bases, the desolvation energy of the exogenous base is the primary contribution limiting the rate of endo deprotonation, while steric accessibility and facile proton movement also contribute. For more bulky bases, steric accessibility can play the dominant role. The significant reduction in these barriers observed in the presence of water has important implications for disfavoring less productive catalytic pathways and increasing catalytic rates.

 Please wait while we load your content...
Please wait while we load your content...