Iridium-mediated C–S bond activation and transformation: organoiridium(iii) thioether, thiolato, sulfinato and thiyl radical compounds. Synthesis, mechanistic, spectral, electrochemical and theoretical aspects†
Abstract
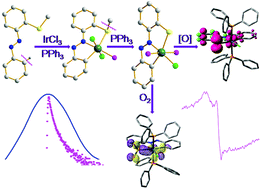
An attractive methodology, single-electron transfer (SET) reductive cleavage of the C–S bond mediated by a metal in the presence of the external stimuli PPh3, has been applied to the kinetically inert IrCl3 in order to synthesize the thiolato complex [IrIII(LS)Cl(PPh3)2] 3 from precursor thioether complexes [IrIII(LSR)Cl2(PPh3)] (R = alkyl) 2. The aforesaid cleavage process in association with (arene)C–H activation furnishes a new class of organosulfur compounds of iridium(III). The thiolato chelate 3 displays a reversible oxidative wave at 0.75 V vs. Ag/AgCl signifying its remarkable nucleophilic character. The high electron density on the thiolato-S vis-à-vis superior nucleophilicity can be envisaged through the formation of a number of S-centered derivatives. This observation has been corroborated with the nature of HOMO in 3, which assumes 49% of S3p. Notably, the facile oxidative nature of 3 makes it an apposite precursor for metal-stabilized thiyl radical species. Indeed, iridium(III)-stabilized 3˙+ can be generated by chemical/electrochemical means. The axial EPR spectra with g ∼ 2.0 along with theoretical analysis of SOMO (S3p 24% + Phπ 43% + dyz 15%) and spin density (ρS = +0.543, ρPh = +0.315, ρIr = +0.151) of one-electron oxidized 3˙+ validate the iridium-stabilized thiyl radical description. This observation suggests that the CNS coordination mode in thiophenolato complex 3 is redox-active. Complex 3 is very prone to S-centered oxidation under normal aerobic conditions to yield metallosulfoxide [IrIII(LSO2)Cl(PPh3)2] 4. The enhanced nucleophilicity of thiolato-S can also be manifested via the smooth S–C bond making process with alkyl halides (R′X, R′ = Me and allyl; X = Br, I) and subsequent formation of thioether complexes of type [IrIII(LSR′)ClX(PPh3)] 5. The organosulfur compounds of iridium(III) exhibit rich spectral properties including luminescence and the origin of these transitions is scrutinized with DFT and TD-DFT methods.

 Please wait while we load your content...
Please wait while we load your content...