Synthesis and structural characterization of silver(i), copper(i) coordination polymers and a helicate palladium(ii) complex of dipyrrolylmethane-based dipyrazole ligands: the effect of meso substituents on structural formation†
Abstract
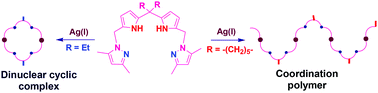
A new class of multidentate dipyrrolylmethane based ditopic tecton, 1,9-bis(3,5-dimethylpyrazolylmethyl)dipyrrolylmethane, containing diethyl (L1) or cyclohexylidene (L2) substituents at the meso carbon atom were readily synthesized in 28–45% yields in two different ways starting from dipyrrolylmethanes. A one dimensional coordination polymer structure ([(L2)Ag][BF4])n was obtained when L2 was treated with AgBF4, whereas the analogous reaction between L1 and AgBF4 afforded the dicationic binuclear metallacycle complex [(L1)2Ag2][BF4]2. In addition, yet another coordination polymeric structure [(L1)CuI]n was obtained from the reaction between L1 and CuI. The analogous reaction of L1 with [Pd(PhCN)2Cl2] afforded the binuclear palladium complex [(L1)2Pd2Cl4] having a double-stranded helicate structure. The observed structural differences are attributed to the effects of the substituents present at the meso carbon atom of the ligand, in addition to the nature of the metal centre, coordination number and the preferred geometry.

 Please wait while we load your content...
Please wait while we load your content...