Correlation between the molecular structure and the kinetics of decomposition of azamacrocyclic copper(ii) complexes†
Abstract
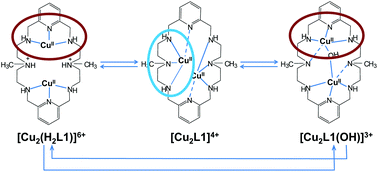
The formation of copper(II) complexes with symmetrical dinucleating macrocyclic ligands containing two either monomethylated (L1) or trimethylated (L2) diethylenetriamine (Medien or Me3dien) subunits linked by pyridine spacers has been studied by potentiometry. Potentiometric studies show that L1 has larger basicity than L2 as well as higher stability of its mono- and binuclear complexes. The crystal structures of L1·6HCl (1), [Cu2(L1)Cl2](CF3SO3)2 (2), [Cu2(L1)(OH)](ClO4)3·3H2O (3) and [Cu(L1)](ClO4)2 (4) show that L1 adopts different coordination modes when bound to copper(II). Whereas in 2, each copper(II) is bound to one Medien subunit and to one pyridine group, in 3 each metal center is coordinated to one 2,6-di(aminomethyl)pyridine moiety (damp) and to one aminomethyl group. The mononuclear complex 4 shows pseudo-octahedral coordination with two weakly coordinated axial nitrogens. Kinetic studies indicate that complex decomposition is strongly dependent on the coordination mode of L1. Upon addition of an acid excess, all the species except [Cu2(L1)]4+ convert very rapidly to an intermediate that decomposes more slowly to copper(II) and a protonated ligand. In contrast, [Cu2(L1)]4+ decomposes directly without the formation of any detectable intermediate. These results can be rationalized by considering that the crystal structures are maintained in solution and that the weakest Cu–N bonds are broken first, thus indicating that kinetic measurements on complex decomposition can be used to provide information about structural reorganizations in the complexes. In any case, complete decomposition of the L1 complexes takes place in a maximum of two kinetically resolvable steps. However, minor changes in the structure of the complexes can lead to drastic changes in the kinetics of decomposition and the L2 complexes decompose with polyphasic kinetics in which up to four different steps associated with the successive breaking of the different Cu–N bonds can be resolved.

 Please wait while we load your content...
Please wait while we load your content...