Tetraphenolate niobium and tantalum complexes for the ring opening polymerization of ε-caprolactone†
Abstract
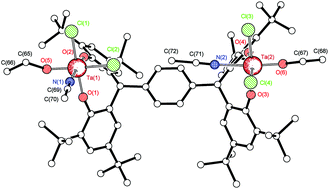
Reaction of the pro-ligand α,α,α′,α′-tetra(3,5-di-tert-butyl-2-hydroxyphenyl-p-)xylene-para-tetraphenol (p-L1H4) with two equivalents of [NbCl5] in refluxing toluene afforded, after work-up, the complex {[NbCl3(NCMe)]2(μ-p-L1)}·6MeCN (1·6MeCN). When the reaction was conducted in the presence of excess ethanol, the orange complex {[NbCl2(OEt)(NCMe)]2(μ-p-L1)}·3½MeCN·0.614toluene (2·3½MeCN·0.614toluene) was formed. A similar reaction using [TaCl5] afforded the yellow complex {[TaCl2(OEt)(NCMe)]2(μ-p-L1)}·5MeCN (3·5MeCN). In the case of the meta pro-ligand, namely α,α,α′,α′tetra(3,5-di-tert-butyl-2-hydroxyphenyl-m-)xylene-meta-tetraphenol (m-L2H4) only the use of [Nb(O)Cl3(NCMe)2] led to the isolation of crystalline material, namely the orange bis-chelate complex {[Nb(NCMe)Cl(m-L2H2)2]}·3½MeCN (4·3½MeCN) or {[Nb(NCMe)Cl(m-L2H2)2]}·5MeCN (4·5MeCN). The molecular structures of 1–4 and the tetraphenols L1H4 and m-L2H4·2MeCN have been determined. Complexes 1–4 have been screened as pre-catalysts for the ring opening polymerization of ε-caprolactone, both with and without benzyl alcohol or solvent present, and at various temperatures; conversion rates were mostly excellent (>96%) with good control either at >100 °C over 20 h (in toluene) or 1 h (neat).
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...