Non-oxido divanadium(iv) and divanadium(v) thiolate complexes with a new type of chalcogenide bridging motif†
Abstract
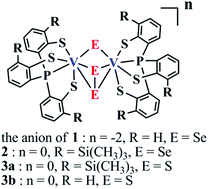
In our effort to study vanadium chalcogenide chemistry, we have synthesized and characterized a class of non-oxido divanadium(IV) and divanadium(V) complexes with chalcogenide and dichalcogenide as bridges. All structures consist of a similar divanadium motif, in which two metal centers are bridged by one μ-chalcogenide and one μ–η2:η2-dichalcogenide, forming a V2(μ-E)(μ–η2:η2-E2) (E = S or Se) core structure. These compounds are [VIV2(PS3)2(μ-Se2)(μ-Se)][PPh4]2 (1), [VV2(PS3′′)2(μ-Se2)(μ-Se)] (2), [VV2(PS3′′)2(μ-S2)(μ-S)] (3a) and [VV2(PS3)2(μ-S2)(μ-S)] (3b) ([PS3]3− = P(C6H4-2-S)3 and [PS3′′]3− = P(C6H3-3-SiMe3-2-S)3). Compound 1 exhibits diamagnetic behavior, indicating strong antiferromagnetic coupling between two d1 centers. Compounds 2 and 3a–b have the highest oxidation states for vanadium ions (+5/+5) among those reported divanadium chalcogenide clusters. The work demonstrates that high-valent divanadium chalcogenide clusters can be obtained with the activation of elemental chalcogens by low-valent vanadium ions.

 Please wait while we load your content...
Please wait while we load your content...