Predicting the reactivity of hydride donors in water: thermodynamic constants for hydrogen†
Abstract
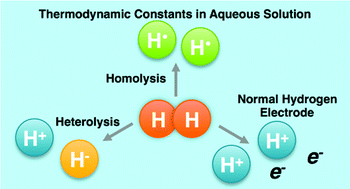
The chemical reactivity of hydride complexes can be predicted using bond strengths for homolytic and heterolytic cleavage of bonds to hydrogen. To determine these bond strengths, thermodynamic constants describing the stability of H+, H˙, H−, and H2 are essential and need to be used uniformly to enable the prediction of reactivity and equilibria. Due to discrepancies in the literature for the constants used in water, we propose the use of a set of self-consistent constants with convenient standard states.

 Please wait while we load your content...
Please wait while we load your content...