Multiple deprotonation of primary aromatic diamines by LiAlH4†
Abstract
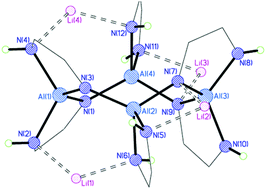
Reaction of LiAlH4 with 1,2-phenylenediamine (1H4) in THF results in formation of the metallocyclic amido-/imido complex [{Al(1H2)}2{Al(1H)2}2][Li(THF)2]4 (3), while in the presence of various Lewis base ligands 1,8-diaminonaphthalene (2H4) gives the amido-(‘ate’) complexes [Al(2H2)2]−[Li(LL′)]+ [L = THF, L′ = PMDETA (N,N,N′,N′,N′′-pentamethyldiethylenetriamine) (4); L = L′ = TMEDA (N,N,N′,N′-tetramethylethylenediamine) (5)]. The latter complexes provide evidence of intermediates in the proposed reaction pathway for formation of the cyclic framework of the tetraanion [{Al(1H2)}2{Al(1H)2}2]4− of 3.

 Please wait while we load your content...
Please wait while we load your content...