Divalent metals can reside on bonds in fullerenes†
Abstract
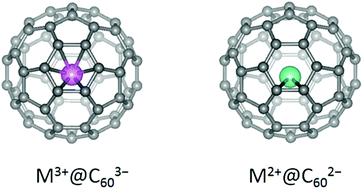
DFT calculations consistently suggest that a lanthanide will sit on either the 6/6 bond inside C60 having a divalent state or the hexagonal center having a trivalent state. Some lanthanides can stay only above the 6/6 bond inside C60 to form stabilized structures, despite the greatly reduced metal–cage coordination numbers. The preference for C–C bonds by a divalent metal has been confirmed by revisiting the structures of Yb@C2v(3)-C80, Yb@Cs(6)-C82 and Yb@C2v(9)-C82, for which the calculations suggest that the Yb atoms are indeed situated above the C–C bonds, close to the reported structures obtained by single crystal XRD experiments. The result will guide the characterization of structures and electronic structures of endohedral metallofullerenes (EMFs), especially C60 mono-EMFs, in the future.


 Please wait while we load your content...
Please wait while we load your content...