Synthesis, characterization and cellular location of cytotoxic constitutional organometallic isomers of rhenium delivered on a cyanocobalmin scaffold†
Abstract
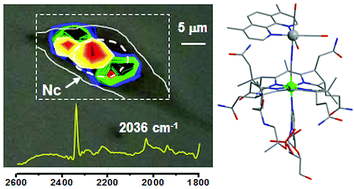
Constitutional isomers of cyanocobalamin adducts based on a fluorescent rhenium tris-carbonyl diimine complex were prepared, characterized and tested against PC-3 cancer cells. The adducts differ only in the relative binding position of the organometallic species which is either bound at the cyano or the 5′-hydroxo group of vitamin B12. When tested for their cytotoxic potency, the species showed IC50 values in the low μM rage. Upon conjugation to the vitamin an energy transfer process causes an extremely low quantum yield of fluorescence emission, making the conjugates unsuitable for fluorescence imaging. However, by exploiting the vibrational signature of the fac-[Re(CO)3]+ core, their cellular distribution was evaluated via FTIR spectromicroscopy.

 Please wait while we load your content...
Please wait while we load your content...