Magnetic and structural properties of dinuclear singly bridged-phenoxido metal(ii) complexes†
Abstract
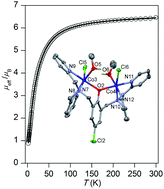
The reaction of a methanolic solution containing the bi-compartmental phenolic ligand 2,6-bis[bis(2-pyridylmethyl)aminomethyl]-4-chlorophenol (LCl-OH) with MCl2·nH2O in the presence of NH4PF6 or NaClO4 afforded the dinuclear bridged-phenoxido dichlorido-metal(II) complexes [Co2(μ-LClO)(H2O)2Cl2][Co2(μ-LClO)(MeOH)2Cl2](PF6)2 (1), [Ni2(μ-LClO)(MeOH)2Cl2]PF6 (2), [Ni2(μ-LClO)(MeOH)(H2O)Cl2]ClO4·1.25H2O (3), [Cu2(μ-LClO)Cl2]PF6·1/2MeOH (4) and [Zn2(μ-LClO)Cl2]PF6·MeOH (5). The complexes were characterized by elemental microanalyses, conductivity measurements, IR and UV-Vis spectroscopy, mass spectrometry and single crystal X-ray crystallography. Each M(II) center within the dinuclear complex cations is octahedrally coordinated in complexes 1–3, and five-coordinated distorted square pyramidal in 4 and 5. Magnetic susceptibility measurements at variable temperature of the complexes 1–4 revealed weak to moderate antiferromagnetic coupling with |J| values = 8.38, 39.0, 30.2 and 0.79 cm−1, respectively. The results of DFT calculations correlate well with the experimentally determined antiferromagnetic coupling and show that the magnetic exchange coupling occurs mainly through the phenoxido bridge M–O–M. Implications of geometry around the central metal ion, M⋯M distance, M–O–M bond angle and overlapping of magnetic orbitals on the magnetic exchange coupling are discussed.


 Please wait while we load your content...
Please wait while we load your content...