Homodinuclear lanthanide {Ln2} (Ln = Gd, Tb, Dy, Eu) complexes prepared from an o-vanillin based ligand: luminescence and single-molecule magnetism behavior†
Abstract
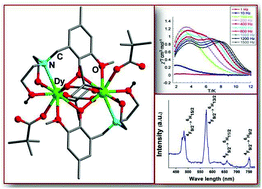
Four dinuclear lanthanide complexes [Gd2 (H2L)2 (µ-piv)2 (piv)2]·2CHCl3 (1), [Tb2 (H2L)2 (µ-piv)2 (piv)2]·2CHCl3 (2), [Dy2 (H2L)2 (µ-piv)2 (piv)2]·2CHCl3 (3) and [Eu2 (H2L)2 (µ-piv)2 (piv)2]·2CHCl3 (4) were synthesized by the reaction of appropriate Ln(III) chloride salts and a multidentate ligand, 2,2′-(2-hydroxy-3-methoxy-5-methylbenzylazanediyl)diethanol (H3L) in the presence of pivalic acid. 1–4 are neutral and are held by two monoanionic, [H2L]− ligands. The two lanthanide ions are doubly bridged to each other via two phenolate oxygen atoms. Both the lanthanide ions are nine coordinated and possess a distorted capped square antiprism geometry. Photophysical studies reveal that Tb3+ (2) and Dy3+ (3) complexes display strong ligand-sensitized lanthanide-characteristic emission. The Tb3+ complex (2) shows a very high overall quantum yield of 76.2% with a lifetime of 1.752 ms. Magnetic studies reveal single-molecule magnet behavior for 3 which shows in its ac susceptibility studies a two-step slow relaxation yielding two effective relaxation energy barriers of ΔE = 8.96 K and 35.51 K.

 Please wait while we load your content...
Please wait while we load your content...