Highly selective colorimetric sensing of Hg(ii) ions in aqueous medium and in the solid state via formation of a novel M–C bond†
Abstract
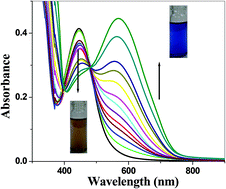
For the first time an easy-to-make receptor 2-chloro-3-(thiazol-2-ylamino)naphthalene-1,4-dione (R1) for highly selective sensing of Hg(II) ions in aqueous solution and in the solid state through the formation of an Hg–C bond was developed. The Hg(II) ion sensing properties of R1 were investigated using UV-Vis, fluorescence and 1H & 13C NMR spectral studies. The results indicated that the receptor selectively senses Hg(II) ions via the formation of a 1 : 1 complex of moderate stability (Ka = 3.5 × 104 M−1). The NMR spectral studies indicated that complexation between R1 and Hg(II) occurs through the formation of an Hg–C bond (after deprotonation), which was confirmed by a single crystal XRD analysis of the product. When Hg(II) was added to a solution of R1 in DMF–water (1 : 9 v/v), a dramatic color change from pale brown to blue was observed, while many common cations and anions did not interfere with the recognition process. The detection limit was 0.3 μM, which is much lower than the permissible limit of Hg(II) in drinking water (0.001 mg L−1) as recommended by the WHO. The simple grinding of R1 with Hg(II) in the solid state also exhibited the same dramatic colour change which is easily detectable visually.

 Please wait while we load your content...
Please wait while we load your content...