Structural and luminescence studies of the new nitridomagnesoaluminate CaMg2AlN3
Abstract
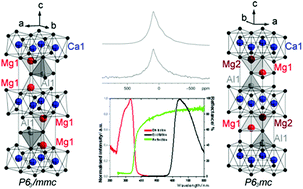
CaMg2AlN3 was synthesized in a closed system by solid state reaction from binary nitrides. Structure refinements based on powder X-ray diffraction data suggested ambiguity about the occupancy of magnesium and aluminum tetrahedral sites. Solid-state 27Al and 25Mg NMR studies were used to adjudicate amongst possible space groups. With reference to projector augmented wave calculations of the quadrupolar coupling constants, the measured values of CQ and the numbers of crystallographically inequivalent Al and Mg sites indicate that CaMg2AlN3 crystallizes in the space group P63/mmc with partial occupancy of the distorted tetrahedral Al site and possibly also mixing of Mg2+ and Al3+ ions on opposite sites. The compound obtained by synthesis with a flux shows orange defect-related luminescence at room temperature.

 Please wait while we load your content...
Please wait while we load your content...