3,3′-Di(pyrazinamoyl)-2,2′-bipyridine: rational ligand design for the self-assembly of a 1-D coordination polymer†
Abstract
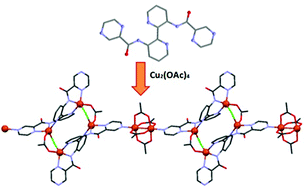
A new ligand, 3,3′-di(pyrazinamoyl)-2,2′-bipyridine, L3H2 has been prepared and is comprised of distinct binding domains capable of facilitating the formation of coordination polymers. Reaction of L3H2 with [Cu2(OAc)4(H2O)2] in the presence of Et3N·HCl affords the complex {[Cu6(L3)2(Cl)2(OAc)6]·2H2O·2MeOH}n (1) in which the [L3]2− anion binds two unique copper ions in a bis-tridentate fashion via amide and pyridyl N atoms. This bimetallic unit is linked to an equivalent unit via a pair of 1,3-acetato and μ-chloro bridges to afford a tetrameric [Cu4(L3)2Cl2(OAc)2] core. Coordination of one of the pyrazole substituents from each ligand to a third copper centre of a rigid paddlewheel [Cu2(OAc)4] unit leads to a unique 1-D polymeric structure. Magnetic studies of 1 reveal that the magnetism can be described in terms of two sets of exchange interactions; strong antiferromagnetic interactions between the two copper centres within the “paddle-wheel” (J/k = −190 K) and a combination of weaker ferro- and antiferro-magnetic interactions within the tetrameric core (J′/k = −5.7 K and J′′/k = +2.1 K).

 Please wait while we load your content...
Please wait while we load your content...