cis-{MoO2}2+ assisted Mannich-type addition of acetylacetonate methine to the azomethine of tridentate Schiff bases: racemic complexes with chiral transformed ligands†‡
Abstract
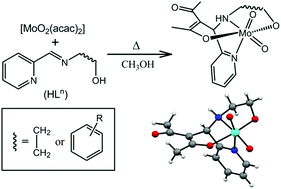
Reactions of [MoO2(acac)2] (acac− = acetylacetonate) with the potential N2O-donor 5,5-membered fused chelate rings forming Schiff bases 2-(2-pyridylaldimine)ethanol (HL1) and 4/5-R-2-(2-pyridylaldimine)phenols (HLn; n = 2–5 for R = H, 4-Cl, 4-Me and 5-Me, respectively) lead to the facile formation of racemic complexes of the general formula cis-[MoO2(acacL1–5)] (1–5) in 80–85% yield. Here, (acacLn)2− represents a chiral N2O2-donor ligand system formed by a novel Mannich-type reaction that involves acetylacetonate and the azomethine fragment of HLn both coordinated to the cis-{MoO2}2+ unit. The characterization of 1–5 has been performed with the help of microanalytical (CHN), spectroscopic (ESI-MS, IR, UV-Vis and 1H- and 13C-NMR) and electrochemical measurements. The molecular structures of all the complexes except for 5 are authenticated by single crystal X-ray crystallography. The Mo(VI) center in each of these analogous complexes is in a distorted octahedral N2O4 coordination sphere assembled by the chiral N2O2-donor transformed ligand (acacLn)2− and the two mutually cis-oriented oxo ligands. In the crystal lattice, each of 1–4 exists as a centrosymmetric discrete dimer via a pair of reciprocal N–H⋯O hydrogen bonds between its enantiomeric pairs.

 Please wait while we load your content...
Please wait while we load your content...