A real-time fluorescent sensor specific to Mg2+: crystallographic evidence, DFT calculation and its use for quantitative determination of magnesium in drinking water†
Abstract
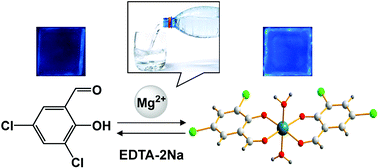
An “off-the-shelf” fluorescence “turn-on” Mg2+ chemosensor 3,5-dichlorosalicylaldehyde (BCSA) was rationally designed and developed. This proposed sensor works based on Mg2+-induced formation of the 2 : 1 BCSA–Mg2+ complex. The coordination of BCSA to Mg2+ increases its structural rigidity generating a chelation-enhanced fluorescence (CHEF) effect which was confirmed by single crystal XRD studies of the BCSA–Mg2+ complex and TD/DFT calculations. This sensor exhibits high sensitivity and selectivity for the quantitative monitoring of Mg2+ with a wide detection range (0–40 μM), a low detection limit (2.89 × 10−7 mol L−1) and a short response time (<0.5 s). It can also resist the interference from the other co-existing metal ions, especially Ca2+. Consequently, this fluorescent sensor can be utilized to monitor Mg2+ in real time within actual samples from drinking water.

 Please wait while we load your content...
Please wait while we load your content...