Redox activity and π bonding in a tripodal seven-coordinate molybdenum(vi) tris(amidophenolate)†
Abstract
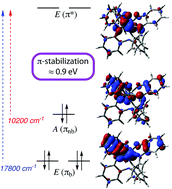
A tris(aminophenol), tris(2-(3′,5′-di-tert-butyl-2′-hydroxyphenyl)amino-4-methylphenyl)amine, MeClampH6, is prepared in three steps from tri-p-tolylamine. The ligand reacts with dioxomolybdenum(VI) bis(acetylacetonate) to form an oxo-free heptadentate complex, (MeClamp)Mo, with a capped octahedral geometry. The molybdenum is formally in the +6 oxidation state, with significant π donation of the amidophenolates, as judged by intraligand bond distances. Two ligand-based oxidations and one metal-centered reduction are observed by cyclic voltammetry. Analysis of the optical spectrum of the compound gives an estimate of the energetic stabilization of the ligand π orbitals by bonding to the molybdenum of approximately 0.9 eV, corresponding to about 40 kcal mol−1 per π bond.

 Please wait while we load your content...
Please wait while we load your content...