Synthesis and characterisation of nickel Schiff base complexes containing the meso-1,2-diphenylethylenediamine moiety: selective interactions with a tetramolecular DNA quadruplex†
Abstract
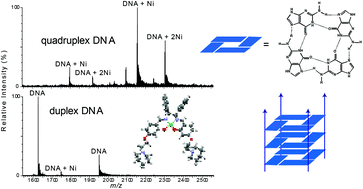
As part of a program of preparing metal complexes which exhibit unique affinities towards different DNA structures, we have synthesised the novel Schiff base complex N,N′-bis-4-(hydroxysalicylidine)meso-diphenylethylenediaminenickel(II) (4), via the reaction of meso-1,2-diphenylethylenediamine and 2,4-dihydroxybenzaldehyde. This compound was subsequently reacted with 1-(2-chloroethyl)piperidine or 1-(2-chloropropyl)piperidine, to afford the alkylated complexes N,N′-bis-(4-((1-(2-ethyl)piperidine)oxy)salicylidine)meso-1,2-diphenylethylenediaminenickel(II) (5) and N,N′-bis-(4-((1-(3-propyl)piperidine)oxy)-salicylidine)meso-1,2-diphenylethylenediaminenickel(II) (6), respectively. These complexes were characterised by microanalysis and X-ray crystallography in the solid state, and in solution by 1H and 13C NMR spectroscopy. Electrospray ionisation mass spectrometry (ESI-MS) was used to confirm the identity of (5) and (6). The affinities of (5) and (6) towards a discrete 16 mer duplex DNA molecule, and examples of both tetramolecular and unimolecular DNA quadruplexes, was explored using a variety of techniques. In addition, the affinity of two other complexes (2) and (3), towards the same DNA molecules was examined. Complexes (2) and (3) were prepared by methods analogous to those which afforded (5) and (6), however 1,2-phenylenediamine was used instead of meso-1,2-diphenylethylenediamine in the initial step of the synthetic procedure. The results of ESI-MS and DNA melting temperature measurements suggest that (5) and (6) exhibit a lower affinity than (2) and (3) towards the 16 mer duplex DNA molecule, while circular dichroism (CD) spectroscopy suggested that none of the four complexes had a major effect on the conformation of the nucleic acid. In contrast, ESI-MS and CD spectroscopy suggested that both (5) and (6) show significant binding to a tetramolecular DNA quadruplex. The results of ESI-MS and Fluorescence Resonance Energy Transfer (FRET) assays indicated that (5) and (6) did not bind as tightly to a unimolecular DNA quadruplex, although both complexes had a major effect on the CD spectrum of the latter. These results highlight that the presence of the meso-1,2-diphenylethylenediamine moiety in metal complexes of this type may provide a general method for instilling selectivity for some DNA quadruplexes over dsDNA.

 Please wait while we load your content...
Please wait while we load your content...