Amino acid derived CuII compounds as catalysts for asymmetric oxidative coupling of 2-naphthol†
Abstract
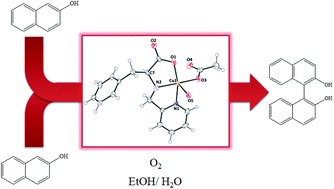
We report the synthesis and characterization of several novel aminopyridine – L-amino acid derived CuII-complexes. The ligands are prepared by a one-pot reductive alkylation of the L-amino acid scaffold and the respective aminopyridine CuII-complexes were obtained by reaction with CuCl2 or Cu(acetato)2. All compounds were characterized by spectroscopic techniques, as well as ESI-MS. Two of the CuII-complexes were characterized by single-crystal X-ray diffraction, one of them, [CuII(L)(CH3COO)] (HL = (S)-3-phenyl-2-(pyridin-2-ylmethylamino)propanoic acid), being the first ever reported aminopyridine-class CuII complex bearing a tridentate N,N,O donor set and a monodentate acetato ligand. The complexes are tested as catalysts in the oxidative coupling of 2-naphthol in organic solvent–water mixtures using dioxygen as the terminal oxidant. The effect of variables such as ligand denticity and substituents, as well as solvent, temperature and oxidant intake, on the overall performance is studied. In general, moderate to low conversions of 2-naphthol to 1,1′-bi-2-naphthol (BINOL) are obtained. The catalysts also showed moderate to low enantioselectivity. Some aspects of the reaction mechanism were elucidated by spectroscopy, electrochemical and theoretical studies. It was found that basic additives are important for activity, but these also increase the formation of secondary oxidation products. The addition of peroxide scavengers such as KI resulted in an increase of conversion, the yield of BINOL and enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...