A salen-type Dy4 single-molecule magnet with an enhanced energy barrier and its analogues†
Abstract
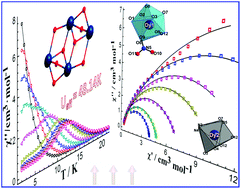
Four isomorphic tetranuclear lanthanide complexes, namely [Ln4(L)2(HL)2(NO3)2(OH)2](NO3)2·4H2O (Ln = Dy (1); Tb (2); Ho (3); Er (4)), constructed using hexadentate salen-type ligand N,N′-bis(3-methoxy-salicylidene)cyclohexane-1,2-diamine, have been isolated. X-ray crystallographic analysis reveals that all of the complexes 1–4 are of discrete tetranuclear structure with a unique {Ln4O8} core in which four lanthanide ions are coplanar in a rhombic frame. There are two crystallographically unequivalent lanthanide ions, that is the Ln1III ion which is nine-coordinated in a monocapped square-antiprismatic geometry of the C4v point group and the Ln2III ion which is eight-coordinated in a distorted bicapped trigonal-prismatic geometry of the C2v point group. Magnetic analysis reveals that complex 1 exhibits two slow magnetic relaxations with the highest energy barrier among the reported tetranuclear salen-type dysprosium SMMs. This further extends the available SMMs of salen-type lanthanide complexes.

 Please wait while we load your content...
Please wait while we load your content...