Intercalation of triethylphosphine oxide bearing a phosphoryl group into Dion–Jacobson-type ion-exchangeable layered perovskites
Abstract
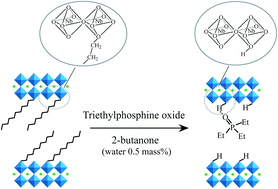
Triethylphosphine oxide [(C2H5)3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O; TEPO] was intercalated into protonated Dion–Jacobson-type ion-exchangeable layered perovskites, HLaNb2O7·xH2O (HLaNb) and HCa2Nb3O10·xH2O (HCaNb), by hydrolysis of their n-decoxy derivatives (C10O-HLaNb or C10O-HCaNb) in the presence of TEPO. The interlayer distances of the products (TEPO/C10O-HLaNb and TEPO/C10O-HCaNb) were smaller than those of the corresponding n-decoxy derivatives, but still larger than those of anhydrous protonated forms of HLaNb and HCaNb. The solid-state 31P NMR signals of the products observed at 94 ppm (TEPO/C10O-HLaNb) and 93 ppm (TEPO/C10O-HCaNb) exhibited large downfield shifts from that of the physisorbed TEPO. IR spectroscopy also showed decreases in the ν(P
O; TEPO] was intercalated into protonated Dion–Jacobson-type ion-exchangeable layered perovskites, HLaNb2O7·xH2O (HLaNb) and HCa2Nb3O10·xH2O (HCaNb), by hydrolysis of their n-decoxy derivatives (C10O-HLaNb or C10O-HCaNb) in the presence of TEPO. The interlayer distances of the products (TEPO/C10O-HLaNb and TEPO/C10O-HCaNb) were smaller than those of the corresponding n-decoxy derivatives, but still larger than those of anhydrous protonated forms of HLaNb and HCaNb. The solid-state 31P NMR signals of the products observed at 94 ppm (TEPO/C10O-HLaNb) and 93 ppm (TEPO/C10O-HCaNb) exhibited large downfield shifts from that of the physisorbed TEPO. IR spectroscopy also showed decreases in the ν(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) band wavenumbers upon the reactions. These results clearly indicate that TEPO is intercalated and interacted with Brønsted acid sites, the surface hydroxyl groups generated via hydrolysis of n-decoxy groups. The difference in ν(P
O) band wavenumbers upon the reactions. These results clearly indicate that TEPO is intercalated and interacted with Brønsted acid sites, the surface hydroxyl groups generated via hydrolysis of n-decoxy groups. The difference in ν(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) band wavenumbers as well as that in gallery heights suggests that TEPO exhibited different orientations with respect to the inorganic layers. The chemical shifts of solid-state 31P NMR signals suggested high Brønsted acidity of HLaNb and HCaNb.
O) band wavenumbers as well as that in gallery heights suggests that TEPO exhibited different orientations with respect to the inorganic layers. The chemical shifts of solid-state 31P NMR signals suggested high Brønsted acidity of HLaNb and HCaNb.

 Please wait while we load your content...
Please wait while we load your content...