Emergence of electrophilic alumination as the counterpart of established nucleophilic lithiation: an academic sojourn in organometallics with William Kaska as fellow traveler†
Abstract
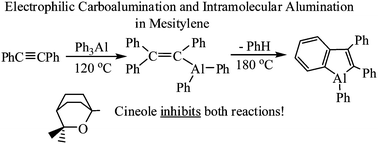
William Kaska pursued doctoral studies with John Eisch in mechanistic organometallic chemistry, first with organolithium reactions at St. Louis University and then at the University of Michigan with organoaluminum reactions. Thereby he revealed the change in mechanism from nucleophilic lithiation and carbolithiation to that of electrophilic alumination, carboalumination and hydroalumination of organic substrates, which reactions were previously observed by Karl Ziegler in his empirical studies of organoaluminum reactions. Our findings were the first mechanistic studies attempting to set such Ziegler chemistry on a modern theoretical basis.
- This article is part of the themed collection: The Carbon–Metal Bond and C–H Metalation

 Please wait while we load your content...
Please wait while we load your content...