Formation, structural characterization, and reactions of a unique cyclotrimeric vicinal Lewis pair containing (C6F5)2P-Lewis base and (C6F5)BH-Lewis acid components†
Abstract
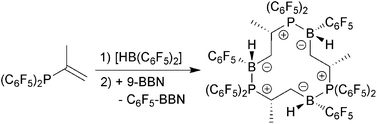
The synthesis of the new vicinal frustrated Lewis pair 5 containing (C6F5)2P-Lewis base and (C6F5)BH-Lewis acid functionality is described. It forms a unique cyclotrimer (5)3 which was structurally characterized by X-ray crystallography and high-resolution solid-state NMR spectroscopy. The relevant NMR Hamiltonian parameters (11B and 31P chemical shielding tensors, 11B quadrupolar coupling tensors, and 31P–11B spin–spin coupling constants) indicate significant intramolecular covalent B⋯P interactions, consistent with results from density functional theory (DFT) calculations. In addition, the 11B/31P and 31P/31P three-spin geometries are accurately reproduced by suitable high-resolution hetero- and homonuclear dipolar NMR experiments. As predicted from the bonding character portrayed by the solid-state NMR results, the cyclotrimer (5)3 possesses only moderate catalytic activity. However, it undergoes an addition reaction with pyridine and hydroboration reactions with benzaldehyde and tert-butylacetylene. The products of the hydroboration reactions form stable adducts with pyridine.

 Please wait while we load your content...
Please wait while we load your content...