Electrode reactions of iron oxide–hydroxide colloids†
Abstract
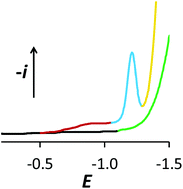
Small-sized FeO(OH) colloids stabilised by sugars, commercially available for the clinical treatment of iron deficiency, show two waves during cathodic polarographic sweeps, or two current maxima with stationary electrodes, in neutral to slightly alkaline aqueous medium. Similar signals are observed with Fe(III) in alkaline media, pH > 12, containing citrate in excess. Voltammetric and polarographic responses reveal a strong influence of fast adsorption processes on gold and mercury. Visible spontaneous accumulation was also observed on platinum. The voltammetric signal at more positive potential is caused by Fe(III)→Fe(II) reduction, while the one at more negative potential has previously been assigned to Fe(II)→Fe(0) reduction. However, the involvement of adsorption phenomena leads us to the conclusion that the second cathodic current is caused again by Fe(III)→Fe(II), of species deeper inside the particles than those causing the first wave. This is further supported by X-ray photoelectron spectra obtained after FeO(OH) particle adsorption and reduction on a gold electrode surface. The same analysis suggests that sucrose stabilising the colloid is still bound to the adsorbed material, despite dilution and rinsing.

 Please wait while we load your content...
Please wait while we load your content...