New Zn2+ coordination polymers constructed from acylhydrazidate molecules: synthesis and structural characterization†
Abstract
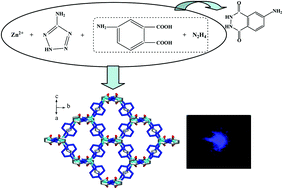
By employing two types of hydrothermal in situ ligand reactions (acylation of N2H4 with aromatic polycarboxylic acids, reduction of 3-nitrophthalhydrazide by N2H4), three new acylhydrazidate-extended Zn2+ coordination polymers [Zn2(3-apth)(atrz)2] (3-apth = 3-aminophthalhydrazidate; atrz = 3-amino-1,2,4-triazolate) 1, [Zn2(4-apth)(atez)2] (4-apth = 4-aminophthalhydrazidate; atez = 5-aminotetrazolate) 2, and [Zn(3-cppth)(H2O)] (3-cppth = 4-(3-carboxyphenoxy)phthalhydrazidate) 3 were obtained. X-ray single-crystal diffraction analysis revealed that (i) compound 1 possesses a 3-D structure. The triazolate molecules link the Zn2+ ions to form a 2-D layer with a (6,3) topology. Then the acylhydrazidate molecule acts as the second linker, extending the (6,3) nets into a 3-D network of compound 1; (ii) compound 2 also exhibits a 3-D structure. The acylhydrazidate molecules first link the Zn2+ ions into a 1-D infinite chain. The tetrazolate molecules propagate further the chains into a 3-D (4,4)-connected net (symbol: (4·64·8)2(42·62·82)); (iii) compound 3 only shows a 1-D chain structure. The photoluminescence analysis indicates that the three title compounds all emit light, especially compound 2 which emits extremely strong blue light. The side group on the phthalhydrazidate molecule plays a crucial role in the emission behaviors of compounds 1–3. At 77 K, the activated compound 2 can adsorb N2 with a capacity of ca. 41.0 cm3 g−1.

 Please wait while we load your content...
Please wait while we load your content...