Chiral multidentate oxazoline ligands based on cyclophosphazene cores: synthesis, characterization and complexation studies†
Abstract
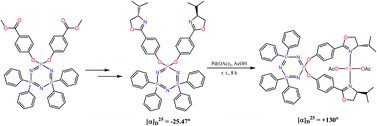
Chiral oxazoline based bi and hexadentate ligands built on cyclophosphazene cores have been synthesized and characterized. (NPPh2)2[NP(m-OC6H4C(O)OCH3)2] (1) was prepared by the reaction of gem-(NPPh2)2(NPCl2) with methyl-3-hydroxy benzoate in the presence of Cs2CO3. Compound 1 was converted to the dicarboxylic acid (NPPh2)2[NP(m-OC6H4C(O)OH)2] (2) by base promoted hydrolysis with KO(t-Bu). The dicarboxylic acid 2 on reaction with oxalyl chloride followed by (S)-(+)-2-amino-3-methyl-1-butanol, triethylamine and mesyl chloride was converted to the C2-symmetric phosphazene based chiral bisoxazoline ligand (NPPh2)2[NP{m-OC6H4(4-iPr-2-Ox)}2] (3) (Ox = oxazolinyl). A similar C2-symmetric bisoxazoline derivative having an oxazoline group attached to the para position of the phenyl ring was also synthesized starting from (NPPh2)2[NP(p-OC6H4C(O)OCH3)2] (4) which was first converted to the dicarboxylic acid (NPPh2)2[NP(p-OC6H4C(O)OH)2] (5) and finally to (NPPh2)2[NP{p-OC6H4(4-iPr-2-Ox)}2] (6) and (NPPh2)2[NP{p-OC6H4(4-Ph-2-Ox)}2] (7) under similar reaction conditions. Reaction of 6 with Pd(OAc)2 in acetic acid at room temperature and with PdCl2(C6H5CN)2 in refluxing benzene resulted in chiral palladium complexes Pd(OAc)2(NPPh2)2[NP{p-OC6H4(4-iPr-2-Ox)}2] (8) and PdCl2(NPPh2)2[NP{p-OC6H4(4-iPr-2-Ox)}2] (9), respectively. The utility of these palladium complexes as chiral catalysts for the asymmetric rearrangement of trichloroacetimidates to trichloroacetamides has been explored. The hexa(methylbenzoate) derivative of cyclophosphazene [PN(OC6H4COOCH3)2]3 (10) on treatment with KO(t-Bu) and H2O gave the hexacarboxylic acid derivative [PN(OC6H4COOH)2]3 (11), which on treatment with oxalyl chloride followed by (S)-(+)-2-amino-3-methyl-1-butanol/(S)-(+)-2-phenylglycinol, triethylamine and mesyl chloride was converted to the C3-symmetric cyclophosphazene based chiral hexaoxazoline ligands [PN{OC6H4(4-iPr-2-Ox)}2]3 (12) and [PN{OC6H4(4-Ph-2-Ox)}2]3 (13). The bis(phebox) derivative of the cyclophosphazene was prepared starting from (NPPh2)2[NP{OC6H3(COOCH3)2}2] (14), by the reaction of gem-Ph4P3N3Cl2 with dimethyl 5-hydroxyisophthalate in the presence of Cs2CO3. Compound 14 was converted to the tetracarboxylic acid (NPPh2)2[NP{OC6H3(COOH)2}2] (15) by base promoted hydrolysis with KO(t-Bu). The tetracarboxylic acid 15 on reaction with oxalyl chloride followed by (S)-(+)-2-amino-3-methyl-1-butanol/(S)-(+)-2-phenylglycinol, triethylamine and mesyl chloride was converted to the bis(phebox) substituted tetraphenylcyclophosphazene derivatives (NPPh2)2[NP{OC6H3(4-iPr-2-Ox)2}2] (16)/(NPPh2)2[NP{OC6H3(4-Ph-2-Ox)2}2] (17). A similar tetra(phebox) derivative was synthesized from (NPPh2)[NP{OC6H3(COOCH3)2}2]2 (18) which was first converted to (NPPh2)[NP{OC6H3(COOH)2}2]2 (19) and further converted to the tetra(phebox) derivative (NPPh2) [NP{OC6H3(4-Ph-2-Ox)2}2]2 (20). All new compounds were characterized by IR, NMR [1H, 13C{1H} and 31P{1H}] and HRMS studies. Compounds 1, 2, 4, 5, 7, 14 and 18 have also been structurally characterized.

 Please wait while we load your content...
Please wait while we load your content...