Proton conductive watery channels constructed by Anderson polyanions and lanthanide coordination cations†
Abstract
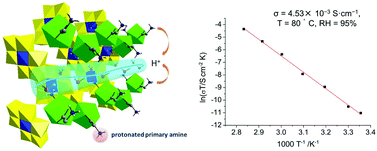
A 3D inorganic–organic hybrid proton conductor, [Sm(H2O)5(CO2CH2NH3)2][Al(OH)6Mo6O18]·10H2O (1), has been synthesized by using coordination cations, [Sm(H2O)5(gly)2]3+ (gly = −CO2CH2NH3+), and polyanions, [Al(OH)6Mo6O18]3− ([AlMo6]). The polyanions ([AlMo6]) and the coordination cations ([Sm(H2O)5(gly)2]3+) stack to form a 3D supramolecular network structure containing 1D channels along the c axis by electrostatic force and H-bonding. Significantly, the 1D channels are water-filled with a high water content (both Sm coordinated and in lattice). Dynamic adsorption measurements were implemented at 1 atm, and 95% relative humidity (RH). The water adsorption amount (6.51 wt% at 25 °C and 5.68 wt% at 80 °C) consistent with the number of lattice water molecules of 1 suggests that the water chains were retained at elevated temperatures (80 °C) under 95% RH. Alternating-current (AC) impedance measurements of 1 reveal an outstanding conductivity for 1 of 4.53 × 10−3 S cm−1 at 80 °C under 95% RH. The activation energy of 1 calculated from the Arrhenius plots of the proton conductivity is 1.09 eV, which indicated that the protons transfer by a vehicle mechanism.

 Please wait while we load your content...
Please wait while we load your content...