Synthesis and physical properties of layered BaxCoO2
Abstract
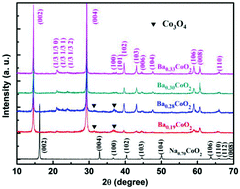
A layered cobaltite BaxCoO2 (x = 0.19, 0.28, 0.30, 0.33) was synthesized by an ion exchange technique from the layered NaxCoO2 precursors. The phase composition and physical properties were investigated. BaxCoO2 is isomorphic to the precursor NaxCoO2. The magnetic susceptibility of BaxCoO2 decreases with increasing barium content and shows a Curie–Weiss-like behavior at temperatures above 50 K. The resistivity is sensitive to the barium content. As the barium content increases from 0.19 to 0.33, a crossover from a semiconducting behavior to a metallic behavior was observed. The Seebeck coefficient of BaxCoO2 is insensitive to the barium content due to the tradeoff effect between the carrier concentration and the Co4+ content, while the thermal conductivity increases with the increasing barium content from 0.19 to 0.33 owing to the ordered state of Ba ions between the CoO2 layers.

 Please wait while we load your content...
Please wait while we load your content...