Behavior of anionic molybdenum(iv, vi) and tungsten(iv, vi) complexes containing bulky hydrophobic dithiolate ligands and intramolecular NH⋯S hydrogen bonds in nonpolar solvents†
Abstract
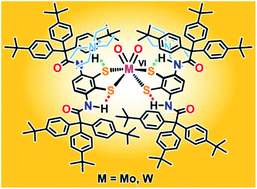
Molybdenum(IV, VI) and tungsten(IV, VI) complexes, (Et4N)2[MIVO{1,2-S2-3,6-(RCONH)2C6H2}2] and (Et4N)2[MVIO2{1,2-S2-3,6-(RCONH)2C6H2}2] (M = Mo, W; R = (4-tBuC6H4)3C), with bulky hydrophobic dithiolate ligands containing NH⋯S hydrogen bonds were synthesized. These complexes are soluble in nonpolar solvents like toluene, which allows the detection of unsymmetrical coordination structures and elusive intermolecular interactions in solution. The 1H NMR spectra of the complexes in toluene-d8 revealed an unsymmetrical coordination structure, and proximity of the counterions to the anion moiety was suggested at low temperatures. The oxygen-atom-transfer reaction between the molybdenum(IV) complex and Me3NO in toluene was considerably accelerated in nonpolar solvents, and this increase was attributed to the favorable access of the substrate to the active center in the hydrophobic environment.

 Please wait while we load your content...
Please wait while we load your content...