The structure and energetics of arsenic(iii) oxide intercalated by ionic azides†
Abstract
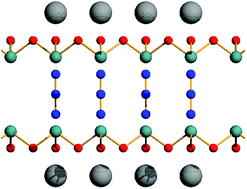
Unprecedented intercalates of arsenic(III) oxide with potassium azide and ammonium azide have been obtained and characterized by single crystal X-ray diffraction. The compounds are built of As2O3 sheets separated by charged layers of cations and azide anions perpendicular to the sheets. The intercalates are an interesting example of hybrid materials whose structure is governed by covalent bonds in two directions and ionic bond in the third one. The obtained compounds are the first examples of As2O3 intercalates containing linear pseudohalogen anions. Periodic DFT calculations of interlayer interaction energies were carried out with the B3LYP-D* functional. The layers are held together mainly by ionic bonds, although the computations indicate that interactions between cations and As2O3 sheets also play a significant role. A comparison of cation and anion interaction energies with neutral As2O3 sheets sheds light on the crystallisation process, indicating the templating effect of potassium and ammonium cations. It consist in the formation of sandwich complexes of cations with crown-ether-resembling As6O12 rings. Raman spectra of both compounds are recorded and computed ab initio and all vibrational bands are assigned.

 Please wait while we load your content...
Please wait while we load your content...