Preparation and properties of a calcium(ii)-based molecular chain decorated with manganese(ii) butterfly-like complexes†
Abstract
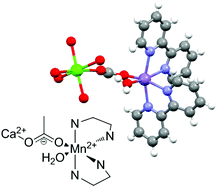
The room temperature reaction of [Mn2O2(bipy)4](ClO4)3 (bipy = 2,2′-bipyridine) with Ca(CHCl2COO)2 in methanol produced a yellow crystalline material. The X-ray determined structure comprises of a multiple calcium(II) carboxylate bridged chain-like structure which is decorated with [Mn(bipy)2(OH2)]2+ subunits. The redox behaviour for the complex in H2O and MeCN is reported. In the latter solvent the oxidation of the manganese ions appears to be facilitated by the presence of the calcium ions. Magnetic susceptibility and low temperature magnetization measurements show that the Mn moment is isotropic, with g = 1.99(1) and S = 5/2, confirming it is in the 2+ oxidation state. A very weak antiferromagnetic interaction is also detected. Frequency-dependent ac measurements evidence slow magnetic relaxation of the Mn(bipy)2 units. Two relaxation mechanisms are identified: a very slow direct process and a faster one caused by the Resonant Phonon Trapping mechanism. This is the first example of field-induced single ion magnet (SIM) behavior in a mononuclear Mn(II) complex.

 Please wait while we load your content...
Please wait while we load your content...