Modulating reactivity in iridium bis(N-heterocyclic carbene) complexes: influence of ring size on E–H bond activation chemistry†
Abstract
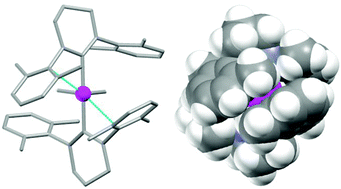
The changes in the steric and electronic properties of N-heterocyclic carbenes (NHCs) as a function of ring size have a profound effect on the reactivity of their late transition metal complexes. Comparison of closely related complexes featuring either a saturated 5- or 6-membered NHC, reveals that the larger ring is associated with an increased propensity towards intramolecular C–H activation, but with markedly lower reactivity towards external substrates. Thus, systems of the type [IrL2(H)2]+ give rise to contrasting chemical behaviour, primarily reflecting the differing possibilities for secondary stabilization of the metal centre by the N-bound aryl substituents: highly labile [Ir(5-Mes)2(H)2]+ can only be studied by trapping experiments, while [Ir(6-Mes)2(H)2]+ is air and moisture stable, and unreactive towards many external reagents. With appropriate substrates, this heightened reactivity can be exploited, and in situ generated [Ir(5-Mes)2(H)2]+ is capable of intermolecular B–H and N–H activation chemistry. In the case of H3B·NMe2H, this affords a rare opportunity to study amine/aminoborane coordination via single crystal neutron diffraction methods.

 Please wait while we load your content...
Please wait while we load your content...