A first-principles investigation of oxygen reduction reaction catalysis capabilities of As decorated defect graphene
Abstract
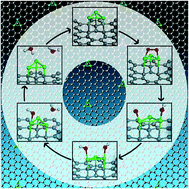
Single and multiple As adatom adsorptions on double vacancy (DV) defect graphene sheets are extensively analyzed using dispersive force corrected density functional theory (DFT). Defect pentagonal and heptagonal bridge sites and the immediate neighborhood of the defect center are found to be most favorable for this purpose. Quantitative analysis of electronic structures revealed the As–C bonding to be mostly ionic in nature with some covalency arising from the overlap of As p states and C p states. For multiple As adatoms adsorption in close vicinity, ionicity of the As–C bonds are found to decrease to support As–As cohesion; the net result of which is manifested as better binding of dioxygen molecule with them and additional weakening of the O–O bond in the adsorbed state. Free energy profile of oxygen reduction reaction (ORR) cycle using multiple As atom adsorbed DV graphene as electrocatalyst predicts high affinity towards four electron process and forbids the formation of H2O2via two electron process. Other traits, such as no intermediate O–O–H formation and high stability of the catalytic system throughout the reaction process indicate As adatoms adsorbed on DV graphene system to be efficient and highly stable as an alternate Pt free ORR electrocatalyst.
- This article is part of the themed collection: Inorganic Chemistry for Renewable Energy Conversion and Storage

 Please wait while we load your content...
Please wait while we load your content...