Scandium versus yttrium{amino-alkoxy-bis(phenolate)} complexes for the stereoselective ring-opening polymerization of racemic lactide and β-butyrolactone†
Abstract
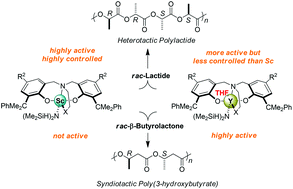
Scandium and yttrium amide complexes Ln{ONXOR1,R2}(N(SiHMe2)2)(THF)n (Ln = Sc, n = 0 or Y, n = 1; X = NMe2 or OMe; R1 = Cumyl or p-Cl-Cumyl; R2 = Me or Cumyl) were prepared by aminolysis of Ln[N(SiHMe2)2]3(THF) with the corresponding tetradentate diamino- or alkoxy-amino-bis(phenol) pro-ligands {ONXOR1,R2}H2. In the solid state and in toluene solution, the scandium complexes are monomeric and 5-coordinated, while the analogous yttrium complexes all bear an extra THF-coordinated molecule and are 6-coordinated. Sc{ONXOR1,R2}(N(SiHMe2)2) complexes are single-site initiators for the ring-opening polymerization (ROP) of racemic lactide but are less active than their yttrium analogues Y{ONXOR1,R2}(N(SiHMe2)2)(THF); also, in contrast to the latter ones, they are inactive in the ROP of the more demanding racemic β-butyrolactone. On the other hand, the scandium amide complexes feature a significantly improved control over the ROP of lactide, yielding PLAs with much narrower molecular weight distributions (ĐM < 1.1 for Sc vs. 1.5–2.0 for Y). The yttrium complex with the very bulky o,p-dicumyl-substituted ligand is more heteroselective than its scandium analogue (Pr = 0.88 vs. 0.83), while the opposite is observed with complexes based on p-methyl-substituted ligands (Pr = 0.50 in toluene or 0.72–0.75 in THF for Y vs. Pr = 0.75–0.83 for Sc in toluene). These reactivity and selectivity trends are rationalized by a much more sterically crowded coordination sphere in scandium than in yttrium complexes.
- This article is part of the themed collection: New Expeditions in Polar Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...